Here are some of the latest news items from Tandem Diabetes Care:
Tandem Source platform launched in the US: On December 12, 2023, Tandem Diabetes Care announced the full U.S. launch of Tandem Source, a new diabetes management platform for customers and healthcare providers. Tandem Source combines the features of Tandem’s legacy t:connect, t:connect HCP, and t:connect Portal offerings with new comprehensive data reporting in one central, scalable platform. Early feedback from users has been positive, and Tandem plans to expand access to other countries in 2024.
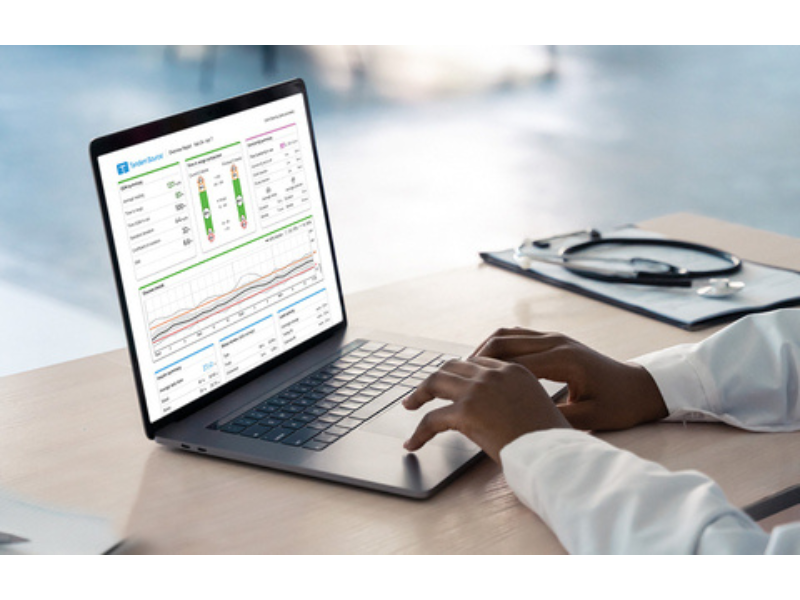 Tandem Diabetes Care Tandem Source platform
Tandem Diabetes Care Tandem Source platform
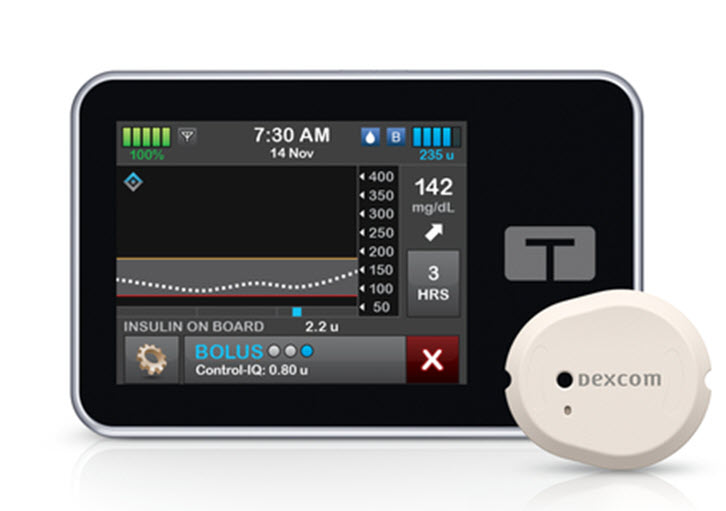
- t:slim X2 Insulin Pump Software with Dexcom G7 CGM Integration: On December 6, 2023, Tandem Diabetes Care launched the t:slim X2 Insulin Pump Software with Dexcom G7 CGM Integration in the US. This integration allows users to seamlessly use the Dexcom G7 CGM sensor with their t:slim X2 insulin pump, providing continuous glucose monitoring and automated insulin delivery.
- Tandem Diabetes Care t:slim X2 Insulin Pump Software with Dexcom G7 CGM Integration
- Unomedical infusion set recall: In October 2023, Unomedical recalled certain infusion sets used with Tandem’s insulin pumps due to a risk of the sets detaching and disrupting insulin delivery. Tandem is working with Unomedical to address the issue and provide replacement sets to affected customers.
These are just a few recent news items from Tandem Diabetes Care. You can visit the company’s website or follow them on social media for more information.
The Unomedical VariSoft infusion set recall is a serious issue for users of Tandem Diabetes Care insulin pumps. Here’s a more detailed breakdown:
What was recalled?
- Product: Unomedical VariSoft Infusion Sets
- Model Numbers: 1002827, 1002828, 1002830
- Lot Numbers: 5388367; 5388357; 5388371; 5388362; 5388368; 5388366; 5388372; 5388376
- Manufacturing Dates: April 1, 2022 to August 1, 2022
Reason for recall:
- Defective connector pieces: Damage to the connector during manufacturing can cause it to unexpectedly detach from the infusion set.
- Disrupted insulin delivery: If the connector detaches, insulin delivery to the patient is interrupted.
- Pump and patient unaware: The insulin pump may not detect the disconnection, and the patient may not know that insulin is not being delivered.
Potential risks:
- Hyperglycemia: Disrupted insulin delivery can lead to high blood sugar levels (hyperglycemia), which can cause serious health complications like diabetic ketoacidosis.
- Diabetic ketoacidosis: This is a life-threatening condition that can occur when the body doesn’t have enough insulin to use glucose for energy and starts breaking down fat instead. Symptoms include nausea, vomiting, abdominal pain, difficulty breathing, and confusion.
- Death: In severe cases, hyperglycemia and diabetic ketoacidosis can lead to death.
What should you do if you have affected sets?
- Stop using the recalled sets immediately.
- Contact Tandem Diabetes Care or Unomedical for replacement sets.
- Tandem has an online form and a hotline (1-877-826-3361) requesting replacements.
- Monitor your blood sugar levels closely.
- Contact your doctor or healthcare provider if you experience any symptoms of hyperglycemia or diabetic ketoacidosis.
Additional information:
- The FDA has classified this recall as Class I, the most serious type of recall, indicating that using the devices may cause serious injury or death.
- Only specific lots and Model Numbers are affected. You can check the full list of affected lot numbers on the FDA website or by contacting Tandem or Unomedical.
- Unomedical and Tandem are working to address the issue and prevent similar recalls in the future.